Citeline Connect Clinical Trial Recruitment Platform Announces Expansion of Reach into all Global Markets
NEW YORK, Oct. 20, 2021 (GLOBE NEWSWIRE) -- CitelineConnect, the all-in-one clinical trial recruitment platform developed by Informa Pharma Intelligence, is expanding its technology and referral network to include EU and global markets.
The expansion comes on the heels of Citeline Connects success in recruiting for Modernas Phase III COVID-19 vaccine trial, particularly for diverse participants. Diane Montross, Senior Director of Patient Recruitment & Retention at Moderna, spoke on the speed and success of the COVE Study recruitment efforts in a presentation at the recent DPharm conference.
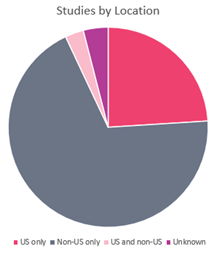
In addition, as the following statistics from Trialtrove indicate, over half of all registered clinical trials involving a drug intervention are conducted outside the US:
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6b9e1c84-9cfa-4405-bc7a-545af1e9f918
| Location | Number of trials | % of total | ||
| US only | 88,305 | 24% | ||
| Non-US only | 257,948 | 69% | ||
| US and non-US | 10,506 | 3% | ||
| Unknown | 14,414 | 4% | ||
| Total | 371,173 | 100% | ||
Based on our overwhelming success recruiting for US-based trial sites, combined with the increased need for trial participants at sites outside the US, we saw an opportunity for Citeline Connect to help more study sponsors meet enrollment goals, said Chris Venezia, Chief Commercial Officer at Citeline Connect. Our robust prescreening process minimizes screen fails, enabling sponsors to recruit faster and more efficiently than ever before.
Citeline Connect has already begun recruiting trial participants globally, and with new consent and platform enhancements the recruitment capabilities have grown to support over 30 countries globally, including the UK, Canada, Austria, Italy, France, Ireland, Germany, Spain, Poland, Hungary, New Zealand, Denmark, Belgium, Portugal, Bulgaria, Israel, Turkey, Taiwan, Australia, Japan, Brazil, Korea, Switzerland, Singapore, Argentina, Georgia, Colombia, Malaysia, India and Russia.
For more information, visit Citeline Connect or contactpharma@informa.com.
About Informa Pharma Intelligence
Informa Pharma Intelligence powers a full suite of analysis products Datamonitor Healthcare, Sitetrove, Trialtrove, Pharmaprojects, Biomedtracker, Scrip, Pink Sheet and In Vivo to deliver the data needed by the pharmaceutical and biomedical industry to make decisions and create real-world opportunities for growth.
With more than 400 analysts keeping their fingers on the pulse of the industry, no key disease, clinical trial, drug approval or R&D project isnt covered through the breadth and depth of data available to customers. For more information, visit pharmaintelligence.informa.com.
Informa Pharma Intelligence PR Contact
Diffusion PR for Informa Pharma Intelligence
informapharma@diffusionpr.com
(213) 318-4500